Hi Beautiful Friends,
Sorry it’s been a minute. Switched hats to direct our fledgling theater company, Backyard Players, in its successful second season. It’s always an undertaking to put on a play in our very own backyard, but the sense of community and the amount of laughter makes the time and effort totally worth it. We couldn’t have done it without some of you within this very Substack community. Thanks!
Coming back to writing felt a little like those first few steps down an escalator that isn’t running. I tried to find a funny meme for this but came up with nothing. If you find one, it would make my day if you left it in the comment section!
If you recall, in Part II (a) we looked at print ads for Premarin from the 1950s, a scary window into an era before modern feminism. The Women’s Movement demanded an end to the grand experiment of untested drugs on women. Researchers began to investigate and find real evidence for the benefits of hormone therapy to replace the unfounded, false promises of eternal youth that had been marketed and funded by Big Pharma.
In this post, I dive into the deep end to look at the seminal research (Nurses’ Health Study, Framingham, PEPI, HERS) on estrogen from 1965 to 2002. If that sounds like a dry pool to you, you’re not wrong. I promise Part III (a) will be way less science and way more celebrity. For those of you who prefer tabloid reading (who doesn’t?), you’ll enjoy the next post in this series more. But without knowing the science behind estrogen, we cannot make an informed choice to use or not to use hormone therapy. At least read about this first study. It’s a little bonkers.
First Trial of Estrogen for Heart Disease — Women Need Not Apply
In 1965, the Coronary Drug Project, the first randomized controlled, double-blind clinical trial to investigate the impact of estrogen on heart disease, looked at 8,341 men with a history of the disease and gave them estrogen (Premarin) or a placebo. It was discontinued when a high dose of 5 mg/day was associated with nonfatal adverse effects and a low dose of 2.5 mg/day demonstrated no positive therapeutic effects.
Now, my math isn’t always good, but with my current patch I’m pretty sure I’m receiving a continuous dose of 0.05 mg/day. The men in this study were given 50-100 times that amount. I’m checking this with my OB because yowza! I’m guessing that different routes of administration impacts bio-availability and absorption and might factor into the discrepancy in dosage. Even oral estrogen when prescribed to women today typically doesn’t exceed 2 mg/day and that is considered a high dose. I’m just picturing men with full bosoms leaping over small buildings and raging at their bus drivers.
If you read my post on the knowledge gap, you may remember that in 1977 the FDA passed a policy recommending a ban of women from clinical drug trials. This exclusion helps explain why randomized, double-blind, placebo controlled trials on estrogen in women didn’t happen until the ban was lifted in 1993. However, it doesn’t explain why the first trial of a hormone produced at much higher levels in women excluded women years before the ban had been enacted by Congress. Although, honestly, I wouldn’t wish doses that high upon any human, or mouse.
Nurses’ Health Study versus Framingham
In 1976 the Harvard School of Public Health began conducting the Nurses’ Health Study, which looked at over 120,000 nurses aged 30-55. This study used information gathered by survey and medical records to bring us a wealth of evidence on many different areas in women’s health: oral contraceptives, smoking, diet, as well as the impact of hormone therapy on cardiovascular disease, cancer, and diabetes.
In 1985, the NHS published the finding that estrogen reduced coronary heart disease by 50%, a finding consistent with those of other observational studies to that point.
In 1980, the Framingham study, which set out to look at long term cardiovascular health in a smaller cohort in Massachusetts, linked estrogen use to as much as 65 percent lower risk of hip fracture, a finding supported in another article in the NEJM.
Yet, in the same year in which the NHS published its findings of heart-protective benefits of estrogen, the Framingham Heart Study, looking at 1,234 women over an 8-year period, found estrogen-users doubled their risk of cardiovascular disease. This opposite finding was a confusing outlier that conflicted with the other studies of that decade. In fact, in a 1991 10-year follow up of the Nurses’ Health Study, their conclusion remained that:
Overall, the age-adjusted risk of major coronary disease among current estrogen users was about half that of women who had never used estrogen, with a relative risk of 0.51 (95 percent confidence interval, 0.37 to 0.70; P<0.0001) (Table 2).
It would be negligent to skip over the NHS finding of an increase in risk of breast cancer among hormone users. The link between hormone therapy and breast cancer is complicated and deserves its own rabbit hole, which I will jump down later. In the meantime, I urge anyone (actually, everyone) to read Estrogen Matters, a carefully researched book that investigates the breast cancer problem in great depth. I’ve also written about this problem in a previous post.
Deborah Copaken, author of the Ladyparts substack, and someone who shares my annoyance at spellcheck failing to identify many words specific to women’s health, including perimenopause, posted recently…
about a 23% reduction of breast cancer risk for those who have had hysterectomies and can take estrogen alone. She also conducted an excellent interview with Dr. Avrum Bluming and Carol Tavris, the authors of Estrogen Matters.
The Limitations of Observational Studies
By the end of the 1990s there were several studies suggesting the protective effect of estrogen not only on bone and heart health, but also on the brain (not to mention what we’ve learned since about the benefits of estrogen on metabolic, vaginal, and emotional health). However, the findings before the 1990s were predominantly from observational studies, so determining cause and effect outside of confounding factors was challenging.
Healthy White Woman Bias
The NHS suffered from the “healthy woman bias,” which posited that its findings were due to the fact that women who took hormones belonged to a subset who were already in better health aside from hormone treatment. The NHS 10-year follow-up report states that those who took estrogen were less likely to have diabetes, more likely to be lean, and more likely to engage in regular vigorous physical activity. The vast majority of participants in the early years of the NHS were middle-to-upper-class white women, yet results were being extrapolated to all women.
Observational studies in the NHS era therefore had two glaring weaknesses: they failed to study a diverse population, and they failed to tease out the chicken-or-the-egg problem. Yet, these studies were the “scientific” evidence we had at the time, and that evidence made estrogen look real good! Since the studies showed a 50% reduction in risk for heart disease, which was and still is the number one killer of women, it made sense that doctors wanted to use estrogen as a blanket prescription for all women of a certain age.
HT the Cure-All
In the nineties, with the evidence for estrogen pointing overwhelmingly to improved and proven bone health, and probable heart health, aided by marketing efforts to sell Premarin as the cure for all chronic illnesses of aging, doctors began over-prescribing it as a preventative measure, regardless of current health status or symptoms.
Here’s the context that helped remove me from the grips of conspiracy thinking to understanding the WHI. The practice of over-prescribing in the wake of Feminine Forever didn’t feel right to some. The times called for more rigorous science to look at the precise impact of long-term hormone therapy and whether it was a reasonable preventative treatment for cardiovascular disease.
Enter the “Gold Standard”
In a randomized control trial (RCT), a participant, unaware of what treatment they are receiving, is given a drug or a placebo to determine the exact impact of the treatment. It has been considered the “gold standard” in evidence-based medicine, although some claim that RCTs too, have their short-comings. They are expensive and difficult to run for long periods of time. You can imagine that if the side effect of receiving the placebo renders one susceptible to hot flashes, maintaining anonymity in this participant group fails. We might also question the ethics of withholding beneficial treatment from a human population. Adherence and drop-out rates can also be problematic. Moreover, as it turns out, the results of RCTs, specifically in the medical literature on HT, are largely similar to those of observational studies.
PEPI- More Good News for HRT
In 1995, PEPI (Postmenopausal Estrogen/Progestin Interventions) was one of the first RCTs for hormone therapy, looking at 875 healthy women aged 45-64. There were five separate arms comparing various treatment regimens. The estrogen tested in this trial was oral Premarin or CEE (conjugated equine estrogen). This trial also looked at two different types of progesterone: MPA (medroxyprogesterone acetate) and oral micronized progesterone.
The study found that estrogen increased “good” cholesterol (HDL) and decreased “bad” (LDL) cholesterol, a result that has also been seen in studies of mice. Though progesterone somewhat muted the positive impact of estrogen, overall even hormones in combination (estrogen plus progesterone) helped improve a woman’s cholesterol levels. Micronized progesterone with estrogen was associated with slightly more benefits to cholesterol profiles, lower blood sugar elevation, and less density in breast tissue than MPA or medroxyprogesterone acetate with estrogen. (The Hormone Decision, p. 290-291)
HERS- A Hint of HRT Danger
In 1998, Wyeth’s confidence in Premarin, their blockbuster drug, led the drug company to fund a large RCT — the Heart and Estrogen Replacement Study (HERS). The objective was to see if estrogen plus progestin therapy decreases the risk of heart disease in postmenopausal women with established coronary heart disease. In 3,000 women, hormone therapy did not improve risk of heart attacks for those who already suffered from coronary artery disease before receiving HRT. In fact, HERS found a “statistically significant increase in heart events in women with known coronary artery disease...” (Estrogen Matters, p. 95)
Turns out the impact of HT varies significantly for older women versus younger women, who are less likely to have progression of heart disease. Interestingly, a 2020 study found that estradiol impacts inflammation differently depending on whether a woman is pre or postmenopausal, reducing certain inflammatory markers before but increasing those same markers after menopause. At the time HERS was conducted, there was no understanding of these differences because they hadn’t yet been investigated.
Another note of interest from HERS is that the increased risk of cardiac events in older women only appeared within the first year of hormone use. Oral estrogen has been seen to increase something called c-reactive protein, an inflammatory marker linked to negative cardiovascular events. In Estrogen Matters, the authors explain that estrogen may increase platelet clumping or cause inflammation in existing plaques within blood vessels and increase bleeding into those plaques, thereby destabilizing them and causing them to burst. However, after the first year, if whatever plaque formation that preexisted before the introduction of HT wasn’t impacted, risk returns to baseline. (Estrogen Matters, p. 96)
Another study from 2002 looking at c-reactive protein showed no increase with transdermal estradiol compared to considerable increase with oral.
Since HERS, there have been numerous studies that help us identify the exact biological impacts, many positive, of hormone therapy, which I’ll address in the final post in this series. It’s difficult not to share that information to balance the bad news that appeared at the turn of this century, but I want to keep us in that era in order to understand what happened next.
In that vein, let’s look at the high hopes for the WHI held by cardiologist Dr. Bernadine Healy, the first and only female director of the NIH hired in 1991. In her 1995 book: Getting the Best Medical Care in a Man’s World: A New Prescription for Women’s Health, she writes glowingly of "HRT, and of the WHI she writes:
In addition to its many other focuses, the WHI will study the long-term effects of HRT in a holistic and interconnected manner. Not only will we learn about the relationship of HRT to heart, brain, bones, and overall health, we will also learn how long it is safe to take HRT, how long a woman needs to take HRT in order to reap its benefits, how HRT needs may change over time, and how they may vary with the overall health, particularly nutritional health, of each woman. Long-awaited definitive answers are on the horizon! (p. 193)
Sadly, not only did the WHI fail to give us these definitive answers, but Healy’s optimism for the future of menopause pales in stark contrast to the same old conversations we’re still having. She believed the boomers would “shatter” old preconceptions, but her chapter on menopause and HRT sounds eerily familiar and current even though she wrote it almost thirty years ago. Sorry for the wah-wah. On the bright side, I see genXers pushing the needle and no way are millenials putting up with some of this sh#$%.
The Study to End All Studies
I’ve already written extensively on the WHI. If you want a short refresher, you can read this:
If you want a detailed description of what went wrong, please check out this post:
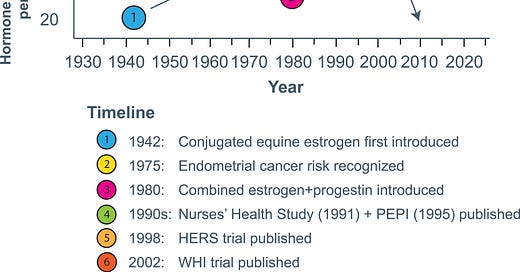
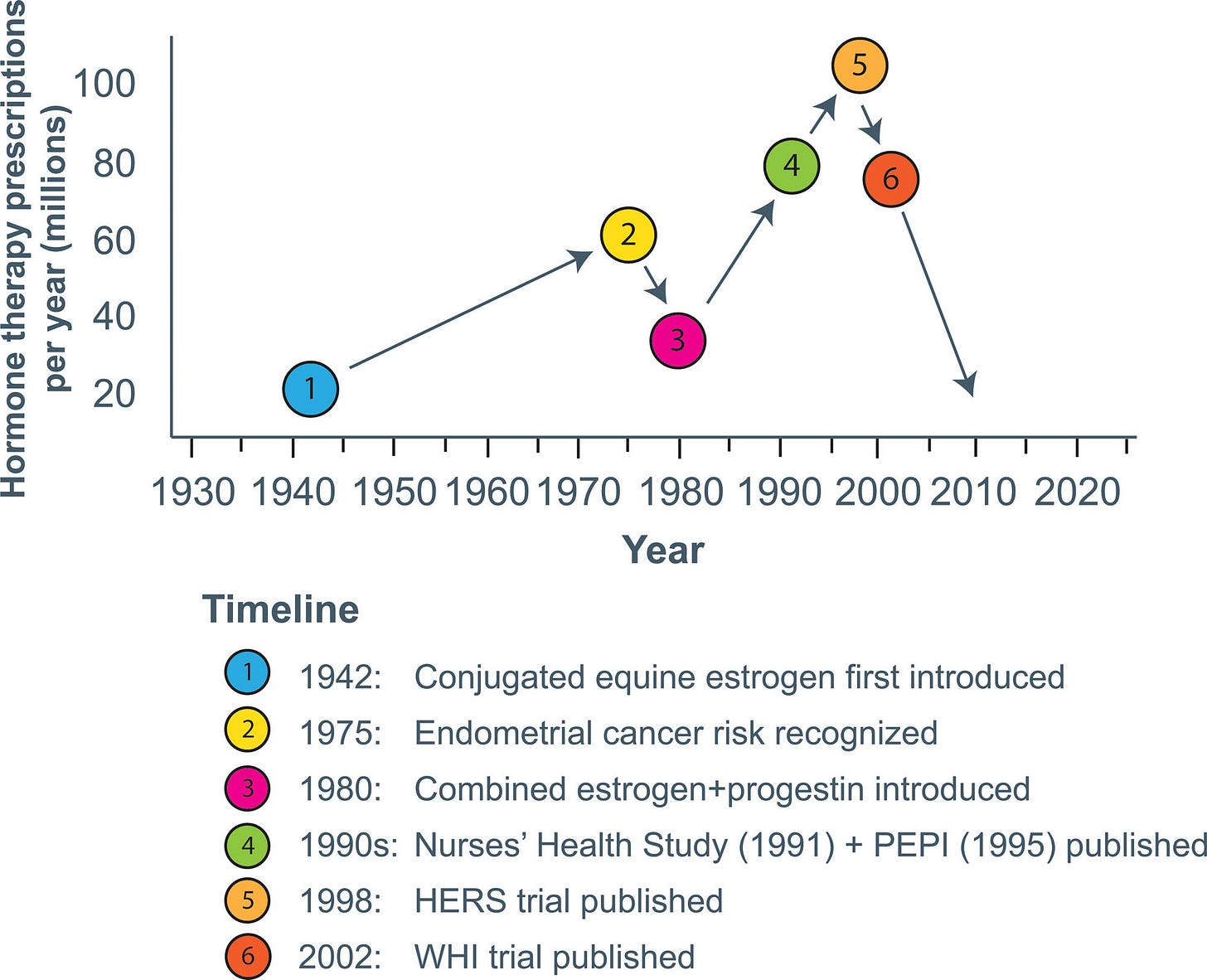
After the bad news of the WHI in July of 2002, the use of hormone therapy decreased rapidly in the US.
Relative to 2001–2002 levels, the prevalence of hormone use among women aged 40 and older was down by 47% in 2003–2004, 72% in 2005–2006, 75% in 2007–2008, and 79% in 2009–2010.
In 2010, use was down to 5% compared to 36.7% when Premarin was at its peak.
The graph at the top of this post charts the ups and downs of Premarin since it’s FDA approval including the final, unrecoverable plummet after the WHI.
Baby with the Bath Water
Knowing what I do now about Premarin, its distasteful marketing, and its abusive manufacturing practices, I personally hope the sales of CEE continue to drop. While CEE may offer unique benefits, the safety and efficacy with newer formulations looks possibly even more promising as demonstrated in studies since the WHI.
Unfortunately, estrogen was the baby that got thrown out with the Premarin bath water in the aftermath of the WHI. Estrogen, a hormone the human body makes and that is responsible for all sorts of important processes from head to toe, is not the bad guy. Yet many women following the WHI press release immediately flushed their HRT down the toilet.
Dangers of Cold Turkey
The negative impact on women quitting their HRT overnight not only landed many in a slew of symptoms, some even had fatal results. A Finnish study showed an increase risk of heart attacks in the year following discontinuation of hormone therapy. One study by the Yale School of Medicine published in the American Journal of Public Health estimated the toll of estrogen avoidance:
The widespread rejection of estrogen therapy after the 2002 Women's Health Initiative (WHI) study has most likely led to almost 50,000 unnecessary deaths over the last 10 years among women aged 50 to 69 who have had a hysterectomy.
Feminine Forever Funded by Pharma
More fuel was fed to the fire in the feminist camp that criticized the medicalization of menopause when Wilson’s son revealed that the drug company that made and sold Premarin, had funded Feminine Forever, paid his parents to lecture on the benefits of hormone therapy, and financed Dr. Wilson’s research foundation.
Oh dear.
Perfect Storm of Distrust
The biggest promoters of hormone therapy were Big Pharma and the sexist doctor it paid to pedal its drug by using oppressive, manipulative messaging. Earlier in the 20th century, the medical establishment mostly denied any real suffering experienced during hormonal transition. Later, doctors broadly prescribed hormone therapy while ignoring cancer risks, leading to thousands of avoidable endometrial cancers before the addition of progesterone.
This backdrop of distrust set us up for the greatest setback for hormone therapy since the discovery of estrogen. Women were left adrift, still suffering symptoms but now in a void without consistent results from the so-called “gold standard” randomized clinical trials.
When Protection Hurts
Like the FDA exclusion law of 1977 that prevented women from entering clinical drug trials, the WHI seemed to prioritize protection over hard clinical data. One of its principle investigators had already showed his hand in a 1996 article in the journal of the American Heart Association, which he entitled “Putting the brakes on the bandwagon.”
The WHI aimed to determine whether long term use of hormone therapy was safe and effective at treating cardiovascular disease. The investigators chose an older population anticipating, correctly, that these women were more likely to have heart attacks. Unfortunately, older women weren’t and still aren’t typically the ones seeking hormone therapy for treatment of hormonal symptoms, just as they weren’t and aren’t the ones who benefit the most. The WHI answered many questions just not the ones Dr. Bernadine Healy and most women considering hormone therapy, were asking.
Consistent with the HERS findings, older women who have already begun to develop heart disease do not fare as well on HT. The group within the WHI that demonstrated the best results were those between the ages of 50-60, a small percentage of the participants. The positive results for this younger subgroup came out in fine print in the long run with much less fanfare than the original scare.
WHA (Won’t. Happen. Again.)
One of the greatest harms of the WHI is that because it was such a large and expensive study, it became the definitive, be-all-and-end-all of studies on hormone therapy. I’ve heard numerous doctors and experts say that we’ll never see a study of that budget, size, and focus again.
HRT to MHT: A Mal Mot Moment
Part III will look at estrogen’s slow crawl back from 2002 to today. In order to reject the medicalization of menopause, and the consequent stigma in labeling the inevitable aging process as “disease,” the term “hormone replacement therapy” has itself been replaced with “menopause hormone therapy.” The idea is that “replacement” suggests hormones are something we need to put back into a woman to keep her a woman.
I don’t believe that postmenopausal women with estrogen levels close to zero are no longer women. In fact, I believe that in the process of leaving reproduction behind women experience a certain license to become more themselves than ever, which, if we identify as woman renders us more woman than ever. However, when I apply my transdermal patch, I am replacing the exact molecule that my body is making less of. Also, I am not currently in menopause, a word I don’t like, which I explain in a previous post. Furthermore, I have a friend who is an internal medicine doctor who prescribes hormone therapy to transgender patients. For all these reasons, I like to drop the “M” from “MHT.” Hormone therapy replaces or introduces molecules into a body in order to improve quality of life and therefore overall health. Menopause, or the specific date of one’s last menstrual cycle is often irrelevant.
What’s on Deck?
In Part III(a): Big Pharma to Big “Natural”, we’ll look at how Big “Natural” stepped in to fill the void left by the WHI for the treatment of symptomatic women. Rearing its head all over social media, Big “Natural” might be currently winning the profit war on menopause, regardless of safety and efficacy in treatment. In this post, I hope to put to rest the problematic use of the word “natural” once and for all. Big “Natural” got its foot in the door by way of celebrity endorsement, a phenomenon that seems to be sprouting up everywhere and deserves some careful consideration.
In Part III (b): Turning the Titanic, we’ll look at the latest data including a recent article in the journal for American Obstetrics and Gynecology, a Medicare study of over 1.5 million women on HT, a meta-analysis from the world’s leading neuroscience menopause expert, and a 20-year follow up of the WHI.
I can barely contain myself!
Stay tuned!







Grateful for your deep dive that lays out all the information so clearly. Thank you!